What Is Ammonium Sulfate?
The chemical compound ammonium sulfate is primarily used in fertilizers but is important in other industries as well. While it isn’t considered highly hazardous to humans, there are some important precautions to take if you're using it.

Chemical Properties
Ammonium sulfate, also called diammonium sulfate or sulfuric acid diammonium salt, is a white crystalline solid with no smell. It tastes salty. The compound dissolves easily in water but will not dissolve in alcohol or acetone. It readily absorbs water, so if it’s exposed to moist air, it will “scab” on the damp surfaces. The chemical formula for ammonium sulfate is (NH₄)₂SO₄. When ammonium sulfate reacts with alkaline substances, it gives off ammonia gas. Finally, ammonium sulfate is a fertilizer that’s sometimes used in making homemade explosives.

Use in Fertilizers
Ammonium sulfate is used most commonly as an artificial fertilizer for alkaline soils. When introduced into damp soil, an ammonium ion is released. This creates a small amount of acid, which lowers the pH balance of the soil. It also contributes nitrogen, which aids in plant growth. It dissolves relatively slowly, which makes it cheaper than some other artificial fertilizers. Ammonium sulfate is also used as an herbicide because it will burn the leaves of plants and either kill them outright or at least weaken them for easy removal.
Other Uses
This compound is used in the production of printed circuit boards. It’s also in flame retardant materials because it lowers the combustion temperature and increases the production of residues or chars. Ammonium sulfate activates yeast, so it helps to get industrially produced bread to rise, and it’s also a general-purpose food additive. Finally, it plays an important role in developing vaccines during the purification process. The DTap vaccine, which protects children from diphtheria, tetanus, and whooping cough, uses ammonium sulfate for this purpose.

Hazards of Use
Ammonium sulfate is potentially dangerous to both people and the environment, so it requires care in its use. It can cause severe irritation and inflammation of the respiratory tract if inhaled. Eating or drinking ammonium sulfate will cause irritation in the gastrointestinal tract like nausea, vomiting, and diarrhea, although it isn’t toxic unless consumed in large quantities. Contact with the skin or eyes will cause irritation, redness, itching, and pain. It may also be a neurotoxin, meaning it can cause confusion and behavioral changes.
Avoiding Exposure
As with any potentially toxic chemical, it’s important to take safety precautions when using ammonium sulfate. Use ammonium sulfate only in a well-ventilated area or with a personal respirator. Wear chemical safety goggles and/or a full face safety shield if dusting or splashing solutions is possible. Also, wearing boots, gloves, and an apron or coveralls made of an impervious material like PVC will prevent skin contact. Any work area should be outfitted with an eyewash station and emergency shower in case of accidental exposure.
While ammonium sulfate is used as a fertilizer and therefore is readily available, it should not be used lightly. Taking basic precautions will allow you to get the benefits of using ammonium sulfate without putting yourself at risk.
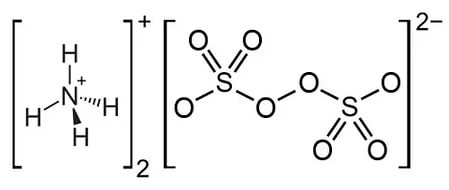
Formula for Ammonium Sulfate
(NH4)2SO4
Properties for Ammonium Sulfate
Molar mass: 132.14 g/mol
Density: 1.769 g/cm3 (20 °C)
Melting point: 235 to 280 °C (455 to 536 °F; 508 to 553 K) (decomposes)
